WE DESIGN, MANUFACTURE AND SUPPLY

WELCOME TO AQUASOLUTIONS INDIA
AQUASOLUTIONS INDIA is an innovative technology and engineering company, jointly working with our Russian partner and positioned to become one of the leading suppliers of water management technology for wide range of applications. Our company focused on developing, designing and commercializing cost effective AQUALYTE systems that produce on site cleaning, sanitizing and disinfecting solutions that safely, effectively, and naturally combat dangerous pathogens.
Our mission is simple: Developing new applications, Consultancy to our clients and supply reliable systems that produce automatically ECO FRIENDLY NATURAL solutions which meet the client requirements and provide the best service with reasonable cost. We stay flexible and dynamic, which allows us to give exactly the amount of personal attention to get the best and most economic solution to the Industry’s specific needs.

We continue to advance our competitive position by expanding our research works and reach across a broad range of industries, markets and new applications.
Dr.Balan
Patriot of ECO FRIENDLY technology
BASIC CONCEPT
The electro activation technology is based on producing in special technical electrochemical systems of metastable (activated) solutions with anomalous physicochemical and catalytic reactivity for their further use in various technological processes in place of traditionally used special chemical agent solutions. Electro activation is effected by simultaneous electrochemical and electro physical action on water in the electric field of the double electric layer of one of the electrochemical system electrodes. Water unipolar electro treatment is accompanied by controlled mass transfer in the inter electrode space with minimal heat generation and with obligatory creation of conditions for the closest contact of each micro volume of liquid under treatment with the dense and/or diffuse part of the double electric layer on the electrode surface where the electric field intensity reaches several million volts/cm.
As a result of electrochemical activation, water transforms into metastable state, which is due to emergence of chemical and physical excitations and is accompanied by directed changes in pH, oxidation-reduction potential and other physical and chemical parameters in a wider range than under chemical regulation.
Chemical excitations in electrochemically activated water and solutions are primarily represented by metastable products of electrochemical decomposition of fresh or low-mineralized water, including free radicals, whose reactivity is based on extremely high electron activity in cathodically treated water (catholyte) or extremely low electron activity in anodically treated water (anolyte). Physical excitations are micro-heterogeneous electrically active structures including gaseous electrochemical reaction products (micro bubbles and clusters), as well as metastable changes in the ion-hydrated sheath structure charged particles.
MODERN DISINFECTANT STUDIES
In the late twentieth century, chemical disinfectants development was aimed at searching ways of activating disinfectants in use rather than creating new agents. For instance, until recently, 6% hydrogen peroxide solution was used for high-level sterilization and disinfection purposes. To decrease its corrosive activity and at the same time maintain or even enhance its biocidal ability, technologies of its application as vapour or gas plasma are presently being developed. Thus, activation of chemical disinfectants is aimed at achieving physical and chemical effect providing high bactericidal efficiency with minimum concentration of active substances, with corrosives or destructiveness against the item’s material as well as toxic effect on human beings being insignificant. In this connection, time of exposure, concentration, temperature and conditions of active substances’ application are principal characteristics of the process of disinfecting a medical item and are the major parameters of any practical method.
Electro activated solutions have been found to contain compounds whose co-presence in a solution is impossible from the standpoint of equilibrial chemical thermodynamics.
In particular, those substances include ozone and active chlorine compounds: Hypochlorous acid, Hypo chlorite-ion, chlorine dioxide and chlorine.
The main factors of EA solutions’ activity are the following:
Electrochemically synthesized alkali in catholyte and acids in anolyte, whose concentration depends on chemical composition and source solution mineralization, and is proportional to specific expenditures of electricity amount in the process of synthesis. The presence of acids in anolyte and alkali in catholyte determines pH values maintained in the solution for a long time, and is a stable factor of EA solution activity. Electrically charged micro-bubbles of electrolysis gases, stabilized by non-compensated electric charges concentrated in gas-liquid interface. Micro-bubble sizes vary between 0.1 and 60 µm and their concentration can reach 106 ml¯¹. The term “micro-bubbles” cannot be considered absolutely exact though these electrically active medium disturbances are really small because the above objects do not feature even relatively stable gas-liquid interface.
In particular, Japanese investigators regard such small-size disturbances as clusters. In EA solutions, micro-bubbles are evenly distributed in the volume and remain intact up to 4000 hours. Micro-bubbles are electrically and chemically active components and can serve as catalysts or inhibitors in oxidation-reduction reactions. Their role is especially considerable in Anolyte solution, since these solutions are saturated with micro-bubbles formed during both anodic and cathodic processes, due to which the active components of these solutions behave as conjugated oxidation-reduction pairs. Electrochemically activated Anolyte demonstrate universal action, i. e. produce damaging effect on all major systemic microbial groups (bacteria, fungi, viruses and protozoan), being harmless for the tissue cells of humans and other higher organisms, i. e. somatic animal cells making up a multi-cellular system.
This is due to essential distinctions in the structure and life conditions of cells representing these life forms. The cells of higher organisms, in the process of their life activity, for instance in oxygenize reactions of P-450 cytochrome functioning, during phagocytosis when microbial cells are adhesive and immobilized, produce and employ a number of highly-active oxidants, such as O2-, O•, 1O2, H2O2, HO2•, HO•, ClO- and others. These cells possess powerful chemical system of anti-oxidant defence preventing toxic effect of such substances on vitally important cell structures. Antioxidant properties of somatic cells are associated with the presence of a strong three-layer lipoproteid membrane containing diene conjugates (-C=C-) with electron-donor properties. Microorganisms do not produce such substances in the process of their life activity and have no powerful anti-oxidant defence systems, that is why electrochemically activated biocidal solutions are highly toxic for them. Biocidal substances in electrochemically activated solutions commonly used as antiseptic or chemotherapy agents are not toxic for human somatic cells, since 50-95% of them are represented by oxidants similar to those produced by the cells of higher organisms.
Chemical potential of molecules and ions in electrically activated biocidal solutions (EA solutions) is much higher than that in non-activated ones. Low mineralization of EA solutions and their better hydration ability increasing cell wall and membrane permeability ensure intensive osmotic and electro osmotic oxidant transfer into inter-cellular medium. Osmotic oxidant transfer through microbial cell membranes is much more vigorous than that occurring through somatic cell membranes due to essential difference between osmotic gradients of these types of cells. Accelerated electro osmotic oxidant transfer is intensified by a multitude of electrically charged micro-bubbles of electrolysis gases creating powerful local electric fields with high level of heterogeneity in areas of contact with biopolymers.
All animal somatic cells are heterotrophic: their trophicity depends on the presence of such nutrient components as glucose, amino acids and fatty acids in extra cellular environment. Biologic well being of a somatic cell is associated with the place it occupies in the process of distributing trophic functions of all multi-cellular system elements (cell backs cell). Animal cell trophic functions subordinate to the inter changeability law. If a cell’s trophicity is disturbed, this disturbance can be corrected by neurotrophic regulations, endocrine regulations, functions of the neighbouring cells, blood nutritive function, etc.
All microbial cells are autotrophic and their nutrition depends on their own energetic activity, i. e. if enzymatic processes in a microbial cell are suppressed, it results in its death since there are no compensatory mechanisms. A microbial cell performs all its trophic functions only with the help of enzymatic reactions. Interaction between microbial cells in their habitat environment is not compensatory; that is, a weak spot of a microbial cell is its autonomy. Maximum employment of the fundamental differences between living creatures of micro- and macro-biologic world is the ideological basis of electrochemically activated biocidal solutions.
In contrast to traditional disinfectant and sterilizing solutions, such as glutaric aldehyde, formaldehyde, chloramines, sodium hypo chlorite, dichlor-izo-cyanurates, per-acetic acid, quaternary ammonium compounds (QAC), heavy metal compounds and other synthetic biocidal substances, the active ingredients of Anolyte are not xenobiotic substances and produce no adverse effect on the organism of man and warm-blooded animals. These substances are inorganic short-lived peroxide compounds usually synthesized in the organism of man and warm-blooded animals by specialized electrochemically active cell enzymes and take part in the processes of neutralizing harmful and foreign substances in the body (phagocytosis).
A metastable peroxide mixture formed in the course of bio-electrochemical reactions in the bodies of man and warm-blooded animals is the most efficient of all commonly used means for microorganism destruction, as it has a multitude of spontaneously realized opportunities to change (irreversibly damage) vitally essential functions of microorganism polymers on the level of electron transfer reactions. In its biocidal action mechanism, electrochemically activated neutral anolyte is similar to gas plasma, and its degradation products are source substances, i. e. low-mineralized water. Upon utilization, they spontaneously degrade forming no toxic xenobiotic compounds and do not require neutralization before being discharged into sewerage system. Activated solutions kills pathogens of bacterial, viral and mycotic etiology and its efficiency considerably exceeds that of such well-known disinfectants as chloramines, sodium hypochlorite-etc.
TECHNOLOGY
The Electro Activation Technology is the process of passing ordinary water with pure salt Solution/sea water through an AQUALYTE reactor where both of these two are mixed and affected by high-intensity electric field. As a result, the dis-balanced changes in water structure occur and water is enriched which results in producing two different Activated solutions* namely Anolyte and Catholyte and leave as one neutral solution, depending on the design of the AQUALYTE reactors.
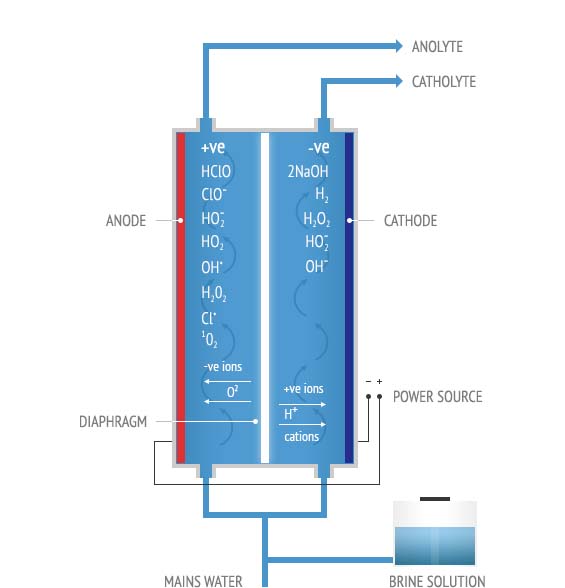
Some of the reactive ions and free radicals species formed in the chambers given below:
Reactive molecules: O3, O3, H2O2, ClO2, HClO, Cl2, HCl, HClO3, NaOH, H2
Reactive ions: H+ , H3O+ , OH- , ClO-
Reactive Free Radicals: HO• , OH2• , O2• , O• , ClO• , Cl• , O2• , ClO•, O2• , O2* , H3O2
The activated solutions develop opposing potentials, the Anolyte having a redox potential of plus 1000mV, while the Catholyte reaches a value of minus 1000mV. The redox potential can be considered a gross indicator of the indicator of the energy incorporated into the respective solutions, likened to the potential built up within thunder clouds relative to the ground, waiting to discharge if anything is available to react with, to neutralize the build-up charge. The solutions can maintain much of their activity for many months or even year. This stability and activity is dependent upon the design of the reactor and power density in use during production of the solutions.
The anolyte has superior sterilizing and disinfectant properties, since the reactive species present in the solution are more effective in destroying microorganisms and organic molecules than chlorine alone. Organic molecules such as pesticides, tannins and phenols, which are of concern in terms of toxicity, color and off-flavors, are effectively oxidized.
The anolyte is an effective means of eliminating organisms of public health concern (E.Coli, Cholera, Dengue, Typhoid, Paratyphoid, Legionaries etc.) from water, via mosquitoes and sewage systems, while simultaneously destroying organic constituents, which are commonly associated with off flavor and color.
The catholye, in turn does not have any sterilizing properties, however, it is a useful in its own right, as the predominance of OH• ions and its reducing power combine effectively to precipitate metal ions out of solution. Hard water, which is typically characterized by high calcium (Ca++), magnesium (Mg++) and iron (Fe++) contents, is softened significantly by precipitating these ions out of solution as illustrated in the following reaction:
Me++ + 2OH- —> MeOH2
MODE OF ACTION:
When the body comes under attack from invading bacteria and viruses, the body's immune system immediately responds by sending increased numbers of a specific white blood cell called a Neutrophil to the invasion site. Once activated, these cells produce substantial quantities of a mixed oxidant solution which is highly effective in eliminating all invading microbes and pathogens. The oxidant that the white blood cells produce is acknowledged to be amongst the most potent natural disinfectants, yet it remains non-toxic to humans, and is highly effective as an antimicrobial agent with rapid action. It is called Hypochlorous acid or HOCl.
HOCl is generated under highly specific electrochemical conditions using a combination of water, salt and electricity. By utilising the specially designed AQUALYTE REACTOR is able to consistently and repeatedly produce HOCl of the highest quality and efficacy, litre after litre. HOCI is extremely effective at eliminating all pathogens and food spoilage microbes including spores.
ACTIVATED SOLUTIONS AND MAIN INGREDIENTS
AQUASOL-C (Anolyte acidic)
AQUASOL-A (Catholyte Alkaline)
AQUASOL-N (Anolyte neutral)
Activated acidic anolyte (pH < 5; ORP =+ 800...+1200mV).
Active ingredients: Cl2; HClO; HCl; HO2•
Activated neutral anolyte (pH 6 ± 1; ORP=+600...+ 900mV).
Active ingredients: HClO; O3; HO•; HO2•
Activated neutral anolyte (pH 7.3 ± 0.5; ORP=+700...+1100mV).
Active ingredients: HClO; ClO-; HO-2; HO2•; H2O2; 1O2; Cl•; HClO2; ClO2; O3; HO•, O•
Activated neutral anolyte (pH 7.7 ± 0.5; ORP=+250...+800mV)
Active ingredients: HClO; ClO-; HO-2; HO2•; HO•; H2O2; 1O; Cl•
Neutral catholyte (pH < 8; ORP= - 200...- 300mV).
Active ingredients: O-2; HO2*; HO-2; H2O2; H•; OH•
Alkaline catholyte (pH > 9.0; ORP= –700...- 820 mv).
Active ingredients: NaOH; O-2; HO2•; HO-2; OH-; OH•; HO-2; O2-2
ACTIVATED SOLUTIONS ARE:
- Environmentally friendly
- Nontoxic
- Not required to have special handling
- Safely disposed in municipal sewage systems
- Fast acting
- Powerful biocide agents
- Used during all stages of disinfections and cleaning
- Applied in liquid, aerosol or frozen forms
- Chemical residue free
- Generated on-site for imminent use
DISINFECTIONS COMPARISIONS
| Disinfectant | Description | Advantages | Limitations |
|---|---|---|---|
| Chlorine | Used in a gaseous state, requires strictest safety measures |
• Efficient oxidant and disinfectant • Efficiently eliminates tastes and odours • Featured with after-effect • Capable of controlling the growth of algae, biological slimes and microorganisms • Decomposes organic contaminants (phenols...) |
• Strict requirements for transportation and storage • Potential danger for health in case of a leak. Formation of disinfection by-products, such as chloroform. The MAC in water will be increased in the near future from 60 mkg/l up to 60 mg/l because there was no proof of direct action of the chloroform on DNA. |
| Hypochlorite | Used in liquid and granulated forms (trade concentration - 10-20%), can be obtained on site, electrochemically |
• Effective against most of pathogen microorganisms • Relatively safe during storage and use • When on-site generated, does not require transportation and storage of chemicals |
• Ineffective against cysts (Giardia, Cryptosporidium) • Loses its activity during long-term storage • Forms trihalomethane. When on-site generated, requires either immediate use or, in case of storage, special measures to purify the initial water from heavy metals ions. When on-site generated, NaCIO solution with the active chlorine concentration less than 450 mg/l does not form chlorates during storage |
| Chlorine dioxide | On-site generation only. The most effective disinfectant and strongest oxidation agent among all chlorine-containing ones |
• Operates in low doses • Does not form chloramines • Does not facilitate trihalomethane formation • Destroys phenols - source of unpleasant taste and odor • Effective oxidant and disinfectant for all types of microorganisms, including cysts, (Giardia, Cryptosporidium) and viruses |
• On-site generation only • Requires transportation and storage of chemicals • In reaction with organic impurities forms nonorganic by-products • Forms chlorates and chlorite ions |
| Chloramine | Formed during the reaction of ammonium with active chlorine. It is used as a disinfectant of a prolonged activity. | Formed during the reaction of ammonium with active chlorine. It is used as a disinfectant of a prolonged activity |
• Weak disinfectant and oxidation agent compared to chlorine • Not effective against viruses and cysts (Giardia, Cryptosporidium) • Considerable dosages and prolonged contact time are required for disinfection • Dangerous for patients using dialyzers, because it is capable of penetrating the dialyzer membrane and effect erythrocytes • Forms nitrogen-containing by-products |
| Ozone | Has been used for several decades in some of European countries for the purpose of disinfection, elimination of color, for the taste and odor control |
• Strong disinfectant and oxidation agent • Very effective against Giardia, Cryposporidium and any other pathogenic micro flora • Facilitates removal of turbidity from water • Removes foreign tastes and odors • Does not form chlorine containing trihalomethanes |
• Forms byproducts, including: aldehydes, ketones, organic acids, bromine-containing trihalomethanes,
(bromoform inclusive), bromates (in presence of bromides): peroxides, brom-acetic acid • Necessitates the use of biologically active filters to remove byproducts • Does not ensure residual disinfection effect • Requires significant initial expenses for the equipment • Considerable expenses for operators` training and installation support • When reacting with organic compounds, ozone disintegrates them into smaller components, which could become a feeding media for microorganisms` growth in water distribution systems |
| Ultraviolet | Exposure of water to UV rays capable of killing various types of microorganisms |
• Does not require storage and transportation of chemicals • Does not form byproducts |
• No residual effect • Not efficient against cysts (Giardia, Cryptosporidium) • Requires considerable expenses for the equipment and technical maintenance • Requires considerable operational (power) expenses • Disinfection activity depends on the water turbidity, its hardness (sediments on the bulb surface), precipitation of organic impurities on the bulb surface, and deviations in the power supply, which effect the wavelength variation |
| AQUASOL | Electrochemical activation of brine solution in a membrane electrolyser |
• Strong disinfect and and oxidation agent • Very effective against all kinds of bacteria and viruses • Highly effective as sporicidal agent • Effectively eliminates bad tastes and odours • Removes biofilms • Significantly less formation of chlorine compounds, halogens and TMT • No toxic by products: chlorites (ClO2) and chlorates (ClO3) • No acute or chronic toxity when diluted in water |
• Need a ventilated area. • Not exposure to sun light directly. |
MATERIAL SAFETY DATA SHEET (Acidic Anolyte)
AQUASOLUTIONS INDIA
85/15,Tirumalai Nagar, Salem –TN – India
aquasol@rambler.ru
Composition and Information on the Ingredients
Acidic Anolyte contains active chlorine compounds such as HCLO and CL2- (C.ac in mg/l) in the range of 0,001-0, 09%. The average/standard amount of active chlorine is ~0, 05%. The solution contains no compounds as per the regulations for toxic compounds (67/548/EWG).
Hazards Identification
The solution is classified as non dangerous accordingly (88/279/EWG)
Main Hazards :
Acidic Anolyte in its strongest form (C.ac >600mg/l) may cause irritation to the eyes, sensitive skin and throat. Where the solution is stored in bottles one should try to smell or inhale the evaporations.
Health effects Eyes:
Acidic Anolyte in its strongest form may cause irritation to the eyes.
Health effects Skin :
Acidic Anolyte in its strongest form may cause slight irritation to sensitive skin or open wounds.
Health effects Ingestion :
Swallowing of the solution in its strongest form may cause irritation to the throat and digestive tract.
Health effects Inhalation :
During generation of Acidic Anolyte, particularly its strong form, unless there is adequate ventilation there may be a build up of fumes which may cause dizziness and nausea.
First aid Measures
Eye contact :
Where irritation occurs flush with cool fresh water
Skin Contact :
Where irritation occurs wash the skin wash with soap and warm water
Ingestion :
Drink cool fresh water to flush through and dilute
Inhalation :
Remove at once to fresh air if dizziness and nausea persist seek medical attention Section 5: Fire Fighting Measures.
Fire Fighting Measures
There are no special requirements for Acidic Anolyte .It is not flammable.
Accidental Release Measures
Personal precautions: None.
Environmental precautions :
The solution is biodegradable and has a limited activation period so there are no potential risks to the environment.
Spillage :
Wipe up with disposable towels there are no special disposal instructions.
Handling and Storage
Handling :
In the area where the solution is being produced there must be good ventilation. Preferably local exhaust ventilation. For those with very sensitive skin it may be advisable to wear gloves.
Storage :
Store in a cool dry ventilated area in sealed plastic containers and ensure the solution is correctly labelled.
Personal Protection and Exposure Control
Engineering control procedures :
Where the solution is being generated on site some engineering solutions should be implemented to prevent the build up of fumes particularly where productions facility has inadequate ventilation. Mechanical fume extraction may be advised in this situation. Documented process, safety controls and personnel protection where necessary, gloves, mask etc.
Respiratory Protection :
Where there is a high risk to fumes build up due to inadequate ventilation in a processing area a respirator should be worn.
Hand protection :
Where service personnel have sensitive skin the strongest solution may cause irritation and therefore protective gloves should be worn.
Eye and facial protection :
There are no requirements.
Body protection :
Normal industrial work wears to avoid exposed skin when handling neat strong solution
Chemical and Physical Properties
- Physical state: Liquid
- Color and Appearance: Clear, transparent liquid (like water)
- Odour: Strong Chlorine odour
- Solubility in water: Completely soluble
- PH-values: 2-5
- Melting-point: 0oC.
- Boiling-point: 100oC.
- Fire-focus: N/A
- Flammability: None
- Explosive: N/A
- Density: app. 1,000 kg.m3
- Steam-pressure: app. 2,330 Pa
Stability and Reactivity
Stability :
Stable under all normal storage conditions.
Materials to avoid :
The solution does not react with other materials
Hazardous decomposition products :
None
Toxicological Information
Acute toxicity :
Not toxic
Irritant-Eyes :
Data for related material suggests this could produce conjunctivitis irritation
Irritant-Skin :
Data for related material suggests this may cause skin irritation
Reproductive and developmental:
None known
Skin contact :
The possibility of allergic sensitization should be considered
Chronic toxicity/Carcinogens :
None
Human Data :
Inhalation may cause respiratory irritation
Environmental Information
Eco toxicity :
Destroys bacteria, viruses, spores and algae
Degradability and Persistence :
Fully Biodegradable
Bio-accumulation :
None
Mobility :
None
Disposal Procedures
There are no special disposal procedures.
Transport procedures
Not classified as hazardous for transport
Regulatory Information
Not listed
Other Information
The information in this document meets the European requirements for safety and health measurements. (91/155/EWG)
The information contained in this document is based on data considered to be accurate at the time of publication
and is given free of charge. It is representative of typical product but batches may exhibit minor variations.
NO warranty is expressed or implied concerning the accuracy of this data.
This information is intended for use in the EC-countries only as different limits may be set in other countries.
MATERIAL SAFETY DATA SHEET (Neutral Anolyte)
AQUASOLUTIONS INDIA
85/15,Tirumalai Nagar, Salem –TN – India
aquasol@rambler.ru
Composition and Information on the Ingredients
Neutral Anolyte contains active chlorine compounds such as HCLO, OCl and CL2 (C.ac in mg/l) in the range of 0,001-0, 09%. The average/standard amount of active chlorine is ~0, 05%. The solution contains no compounds as per the regulations for toxic compounds (67/548/EWG).
Hazards Identification
The solution is classified as non dangerous accordingly (88/279/EWG)
Main Hazards :
Neutral Anolyte in its strongest form (C.ac >600mg/l) may cause irritation to the eyes, sensitive skin and throat. Where the solution is stored in bottles one should try to smell or inhale the evaporations.
Health effects Eyes :
Neutral Anolyte in its strongest form may cause irritation to the eyes.
Health effects Skin :
Neutral Anolyte in its strongest form may cause slight irritation to sensitive skin or open wounds.
Health effects Ingestion :
Swallowing of the solution in its strongest form may cause irritation to the throat and digestive tract.
Health effects Inhalation :
During generation of Neutral Anolyte, particularly its strong form, unless there is adequate ventilation there may be a build up of fumes which may cause dizziness and nausea.
First aid Measures
Eye contact :
Where irritation occurs flush with cool fresh water
Skin Contact :
Where irritation occurs wash the skin wash with soap and warm water
Ingestion :
Drink cool fresh water to flush through and dilute
Inhalation :
Remove at once to fresh air if dizziness and nausea persist seek medical attention
Section 5: Fire Fighting Measures.
Fire Fighting Measures
There are no special requirements for Neutral Anolyte .It is not flammable.
Accidental Release Measures
Personal precautions: None.
Environmental precautions :
The solution is biodegradable and has a limited activation period so there are no potential risks to the environment.
Spillage :
Wipe up with disposable towels there are no special disposal instructions.
Handling and Storage
Handling :
In the area where the solution is being produced there must be good ventilation. Preferably local exhaust ventilation. For those with very sensitive skin it may be advisable to wear gloves.
Storage :
Store in a cool dry ventilated area in sealed plastic containers and ensure the solution is correctly labelled.
Personal Protection and Exposure Control
Engineering control procedures :
Where the solution is being generated on site some engineering solutions should be implemented to prevent the build up of fumes particularly where productions facility has inadequate ventilation. Mechanical fume extraction may be advised in this situation.Documented process, safety controls and personnel protection where necessary, gloves, mask etc.
Respiratory Protection :
Where there is a high risk to fumes build up due to inadequate ventilation in a processing area a respirator should be worn.
Hand protection :
Where service personnel have sensitive skin the strongest solution may cause irritation and therefore protective gloves should be worn.
Eye and facial protection :
There are no requirements.
Body protection :
Normal industrial work wears to avoid exposed skin when handling neat strong solution.
Chemical and Physical Properties
- Physical state: Liquid
- Color and Appearance: Clear, transparent liquid (like water)
- Odour: Chlorine odour depending on strength of the solution
- Solubility in water: Completely soluble
- PH-values: 7, 5 - 8, 5
- Melting-point: 0oC.
- Boiling-point: 100oC.
- Fire-focus: N/A
- Flammability: None
- Explosive: N/A
- Density: app. 1,000 kg.m3
- Steam-pressure: app. 2,330 Pa
Stability and Reactivity
Stability :
Stable under all normal storage conditions.
Materials to avoid :
The solution does not react with other materials
Hazardous decomposition products :
None
Toxicological Information
Acute toxicity :
Not toxic
Irritant-Eyes :
Data for related material suggests this could produce conjunctivitis irritation
Irritant-Skin :
Data for related material suggests this may cause skin irritation
Reproductive and developmental :
None known
Skin contact :
The possibility of allergic sensitization should be considered
Chronic toxicity/Carcinogens :
None
Human Data :
Inhalation may cause respiratory irritation
Environmental Information
Eco toxicity :
Destroys bacteria, viruses, spores and algae
Degradability and Persistence :
Fully Biodegradable
Bio-accumulation :
None
Mobility :
None
Disposal Procedures
There are no special disposal procedures.
Transport procedures
Not classified as hazardous for transport
Regulatory Information
Not listed
Other Information
The information in this document meets the European requirements for safety and health measurements. (91/155/EWG)
The information contained in this document is based on data considered to be accurate at the time of publication and is given
free of charge. It is representative of typical product but batches may exhibit minor variations.
NO warranty is expressed or implied concerning the accuracy of this data.
This information is intended for use in the EC-countries only as different limits may be set in other countries.
MATERIAL SAFETY DATA SHEET (Catholyte)
AQUASOLUTIONS INDIA
85/15,Tirumalai Nagar, Salem –TN – India
aquasol@rambler.ru
Composition and Information on the Ingredients
Catholyte contains hydroxyl and hydroxyl radicals such as NaOH, H202 and OH-. The solution contains no compounds as per the regulations for toxic compounds (67/548/EWG).
Hazards Identification
The solution is classified as non dangerous accordingly (88/279/EWG)
Main Hazards :
Catholyte in its strongest form may cause irritation to the eyes, sensitive skin and throat.
Health effects Eyes :
Catholyte in its strongest form may cause irritation to the eyes.
Health effects Skin :
Catholyte in its strongest form may cause slight irritation to sensitive skin or open wounds.
Health effects Ingestion :
Swallowing of the solution in its strongest form may cause irritation to the throat and digestive tract.
Health effects Inhalation :
During generation of Catholyte, particularly its strong form, unless there is adequate ventilation there may be a build up of fumes which may cause dizziness and nausea.
First aid Measures
Eye contact :
Where irritation occurs flush with cool fresh water
Skin Contact :
Where irritation occurs wash the skin wash with soap and warm water
Ingestion :
Drink cool fresh water to flush through and dilute
Inhalation :
Remove at once to fresh air if dizziness and nausea persist seek medical attention
Section 5 : Fire Fighting Measures.
Fire Fighting Measures
There are no special requirements for Catholyte .It is not flammable.
Accidental Release Measures
Personal precautions :
None.
Environmental precautions :
The solution is biodegradable and has a limited activation period so there are no potential risks to the environment.
Spillage :
Wipe up with disposable towels there are no special disposal instructions.
Handling and Storage
Handling :
In the area where the solution is being produced there must be good ventilation. Preferably local exhaust ventilation. For those with very sensitive skin it may be advisable to wear gloves.
Storage :
Store in a cool dry ventilated area in sealed plastic containers and ensure the solution is correctly labelled.
Personal Protection and Exposure Control
Engineering control procedures :
Where the solution is being generated on site some engineering solutions should be implemented to prevent the build up of fumes particularly where productions facility has inadequate ventilation. Mechanical fume extraction may be advised in this situation.Documented process, safety controls and personnel protection where necessary, gloves, mask etc.
Respiratory Protection :
Where there is a high risk to fumes build up due to inadequate ventilation in a processing area a respirator should be worn.
Hand protection :
Where service personnel have sensitive skin the strongest solution may cause irritation and therefore protective gloves should be worn.
Eye and facial protection :
There are no requirements.
Body protection :
Normal industrial work wears to avoid exposed skin when handling neat strong solution.
Chemical and Physical Properties
- Physical state: Liquid
- Color and Appearance: Clear, transparent liquid (like water)
- Odour: No chlorine smell
- Solubility in water: Completely soluble
- PH-values: 9-13
- Melting-point: 0oC.
- Boiling-point: 100oC.
- Fire-focus: N/A
- Flammability: None
- Explosive: N/A
- Density: app. 1,000 kg.m3
- Steam-pressure: app. 2,330 Pa
Stability and Reactivity
Stability :
Stable under all normal storage conditions.
Materials to avoid :
The solution does not react with other materials
Hazardous decomposition products :
None
Toxicological Information
Acute toxicity :
Not toxic
Irritant-Eyes :
Data for related material suggests this could produce conjunctivitis irritation
Irritant-Skin :
Data for related material suggests this may cause slight skin irritation
Reproductive and developmental:
None known
Skin contact :
The possibility of allergic sensitization should be considered
Chronic toxicity/Carcinogens :
None
Human Data :
Inhalation may cause respiratory irritation
Environmental Information
Eco toxicity:
Degradability and Persistence: Fully Biodegradable
Bio-accumulation: None
Mobility: None
Disposal Procedures
There are no special disposal procedures.
Transport procedures
Not classified as hazardous for transport
Regulatory Information
Not listed
Other Information
The information in this document meets the European requirements for safety and health measurements. (91/155/EWG)
The information contained in this document is based on data considered to be accurate at the time of publication and is given
free of charge. It is representative of typical product but batches may exhibit minor variations.
No warranty is expressed or implied concerning the accuracy of this data.
This information is intended for use in the EC-countries only as different limits may be set in other countries.
Frequently Asked Questions
What chemicals are used to produce AQUASOL solutions?
They are produced using a 0.5 to 1% solution of water and salt (NaCl ) plus electricity.
What does an AQUALYTE generators do?
The generator produces a 100% biodegradable, non-toxic liquid that can replace harsher chemical formulations.
What liquids the generators produce?
The generators produce three basic liquids: Acidic Anolyte, Neutral Anolyte and Catholyte.
What will the liquids do?
The Anolytes are guaranteed to destroy all fungi, spores, bio film, bacteria and viruses in seconds. Total microbial control. The Catholyte is a mild cleaning agent and also works like bio stimulant agent.
What is Acidic Anolyte?
Acidic Anolyte is an acid equivalent. It holds a pH value around 2-3 and can replace chemical agents where an acid agent is used. It is guaranteed to yield better results and be more cost efficient.
What is Catholyte?
Catholyte is a caustic soda equivalent. It holds a pH around 11-13 and can replace chemical agents where caustic soda is used more efficiently and at a far less cost then any chemical formulation. With Catholyte processes like CIP can be accomplished using cold water yield the same results as using caustic soda in warm water.
What is Neutral Anolyte?
Neutral Anolyte is a biocidal agent holds a pH of around 7.5-8.5 and can replace chemical formulations used for rinsing and other tasks where acidity might be a liability.
What are the active agents in the liquids?
The Anolytes consists of 99.6% water, 0.26% salt and 0.05-0.06% hypochlorous acid, hypochlorite ion, hydrochloric acid with a touch of chlorine. The generators contain one (or more) special electrolyte cells that charge the water with an electrical charge. The concentrate of liquid holds around 800 - 1,200 mV of charge (ORP), depending on whether it is acidic or neutral anolyte.
What is so special about this electrolyte cell compared to other similar products?
Low power, low salt consumption (3- 4gm/lit activated solutions), High quality Nobel metals alloys with fine coatings, hence the end product is purely concentrated with less sodium which can be stored for long time.
What is the life expectancy of the AQUALYTE Cells?
Our product line ships with a 1year factory warranty, although the cells are expected to have a life time of 3 to 5 years, after which only the cells need be replaced.
How much liquid can the units produce?
It depends of the unit. We have units that produce from 60 to 100,000 liters a day.
What does the unit cost and what are the running costs?
There are no off-the-shelf prices. All of the units are customized for their intended use. Prices are estimated to range from starting from €7,000 depending on the type of unit and the application use. Once the unit is installed, then the only running costs are ordinary water, edible salt and electricity!
How much do I need to dose in my application?
Each application must be well studied and apply the solutions according to the required result. We will consult our clients.
Products
The below listed products(units) are in our serial production with 60, 120,200, 250, 500, 1000 and more lit/hrs.
We are mainly manufacturing customized units for particular application of our customer. Hence we can develop machine with required parameters to meet the customer need. Please contact us with your specific parameters and we will design for you.
























